Oleg V. Mosin1
1
Department of Biotechnology, M. V. Lomonosov State Academy of Fine Chemical Technology, Vernadskogo Prospekt 86, 117571, Moscow, Russia
1. SUMMARY
The role of deuterium in molecular evolution is most interesting question of nowdays science comprises two points mainly: the evolution of deuterium itself as well as the chemical processes going with participation of deuterium. It is believed the big bang produce the universe that was much denser and hotter than it is now and made almost entirely of two main elements - hydrogen and helium. Deuterium itself was made only at a second stage of the beginning of the universe, namely through the collision of one neutron with one proton at a temperature of about one billion degrees; furthemore the two formed deuterons in turn stuck together into helium nuclei, which contain two protons and two neutrons. It is considered, that during the formation of helium nuclei, almost all the deuterons combined to form helium nuclei, leaving a tiny remant to be detected today so that only one in 10.000 deuterons remained unpaired.
Thus, deuterium serves as a particularly important marker. The quantity of deuterium in contemporary nature is approximately small and measured as no more than 0.015% (from the whole number of hydrogen atoms) and depends strongly on both the uniformity of substance and the total amount of matter formed in course of early evolution. One may suggest, that the very reliable source of producing of deuterium theoretically may to be the numerical explosions of nova stars, but deuterium itself is very readily destroyed in those stars. If it was so, perhaps this was the answer to the question why the quantity of deuterium increased slitely during the global changes of climate for worming conditions.
The second point is the chemical processing of deuterium as a result of this the 2
H2
O on the first hand may be formed from gaseous deuterium and atomic oxyden at very high temperature. Pretty interesting with chemical point of view seems our own idea proposed recently about the possible small enrichment of primodial environment with 2
H2
O. We supposed, that this fact if really existed, may be conditioned by a powerful electrical discharges taken place in premodial atmosphere laking the natural shield of ozone and may be resulting in electrolysis processes of H2
O, e.g. those ones are now used for the enrichment of 2
H2
O. But the realization of this process with practical point of view seems unlikely. Nevertheless, if such process has really occured, the some hydrophobic effects of 2
H2
O as well as chemical isotopic effects should be taken into account while discussing the chemico-physical properties of primodial environment. Perhaps, it is also a big practical interest to study the properties of fully deuterated membraine structures composed for example from fully deuterated lipids and proteins. Either way or not, the model of deuterium evolution provides a framework for predicting the biochemical consequences of such new fascinating ideas.
SUMMARY:
Deuterium (2
H), the hydrogen isotope with nuclear mass 2, was discovered by Urey
. In the years immediately following this discovery, there developed a keen interest in development of methods for uniform biological enrichment of a cell with 2
H, that may be best achived via
growing of an organism on medium with high content of 2
H2
O (99% 2
H), which since yet resulted in a miscellany of rather confusing data (see as an example Katz J., Crespy H. L. 1972
).
The main resolute conclusion that can be derived from the most competent and comprehensive of the early studies is that high concentrationsof 2
H2
O are incompatible with life and reproduction and furthemore could even causing even lethal effects on a cell. However, today a many cells could be adapted to 2
H2
O either via
employing a special methods of adaptation which of them we shall describe above, or using selected (or/and resistent to 2
H2
O) strains of bacterial and other origin.
In this connection the main interesting question arises-what is the nature of this interesting phenomenon of biological adaptation to 2
H2
O and what is the role of life important macromolecules (particularly DNA, individual proteins, and/or enzymes) in this process? It is seems very likely, that during adaptation to 2
H2
O the structure and conformation of [U -2
H]labeled macromolecules undergoing some modifications that are more useful for the working in 2
H2
O-conditions. Unfortunately, there are a small number of experiments carried out with fully deuterated cells, that could confirmed that during the growth on 2
H2
O [U-2
H]labeled macromolecules with difined isotopical structures and conformations are formed, so that a discussion about the role of deuterium on the structure and the conformation of [U-2
H]labeled macromolecules in course of biolodical adaptation to 2
H2
O is still actual through more than four decades of years after the first description of the biological consequences of hydrogen replacement by deuterium.
To further discuss the matter, we should distingueshed mainly three aspects of biological enrichment with deuterium: chemical, biological and biophysical aspects, all of them are connected in some way with the structure of [U -2
H]labeled macromolecules. Theoretically, the presence of deuterium in biological systems certainly could be manifested in more or less degree by changes in the structure and the conformation of macromolecules. Nevertheless, it is important namely what precise position in macromolecule deuterium ocupied and dipending from that the primary and secondary isotopic effects are distingueshied. For example, most important for the structure of macromolecule the hydrogen (deuterium) bonds form between different parts of the macromolecule and play a major part in determining the structure of macromolecular chains and how these structures interact with the others and also with 2
H2
O environment. Another important weak force is created by the three-dimentional structure of water (2
H2
O), which tends to force hydrophobic groups of macromolecule together in order to minimize their disruptive effect on the hydrogen (deuterium)-bonded network of water (2
H2
O) molecules.
On the other side the screw parameters of the proton helix are changed by the presence of deuterium so that ordinary proteins dissolved in 2
H2
O exhibit a more stable helical structure (Tomita K., Rich A., et all., 1962
). While 2
H2
O probably exerts a stabilizing effect upon the three-dimentional hydrogen (deuterium)-bonded helix via
forming many permanent and easily exchangeable hydrogen (deuterium) bonds in macromolecule in the presence of 2
H2
O (as an example the following types of bonds -COO2
H; -O2
H; -S2
H; -N2
H; N2
H2
et.), the presence of nonexchangeable deuterium atoms in amino acid side chains could only be synthesized de novo as the species with only covalent bonds -C2
H, causes a decrease in protein stability.
These opposing effects do not cancel with the case of protein macromolecule, and fully deuteration of a protein often results in the destabilization. As for the deuteration of DNA macromolecule, today there are not reasonable considerations that such negative effect of 2
H2
O on the structure and function is really existiting. Nevertheless, deuterium substitution can thus be expected to modify by changes in the structure and the conformation of both [U- 2
H]labeled DNA and protein, not only the reproductionl and division systems of a cell, and cytological or even mutagenical alterations of a cell, but to a greater or lesser degree of an order of a cell.
It should be noted, however, that not only these functions but also the lipid composition of cell membrane are drastically changed during deuteration. The lipid composition of deuteriated tissue culture cells has been most complitely investigated by a certain scientists (Rothblat et all., 1963, 1964).
As it is reported in these articles mammalian cells grown in 30% (v/v) 2
H2
O contain more lipid than do control cells. THe increase in the lipids of 2
H2
O grown cells is due primarily to increased amounts of triglycerids and sterol esters. Radioisotope experiments indicate that the differens are due to an enhanced synthesis of lipid. Monkey kidney cells grown in 25% (v/v) 2
H2
O and or irradiated with X-rays likewise showed increases of lipid. The 2
H2
O grown cells contained more squalene, sterol esters, sterols, and neutral fat than did either the control of X-irradiated cells. Phospholipid levels were equal for all groups of cells. Thus the effects of 2
H2
O on lipid synthesis are qualitatively quite similar to those of radiation damade. An interisting observation that deserves further scrutiny relates to the radiation sensitivity of deuterated cells. Usually, cells grown and irradiated in 2
H2
O shown much less sensivity to radiation than ordinary cells suspended in water. Suspension of ordinary cells in 2
H2
O did not have any effect on the reduced sensitivety became apparent.
A serious alteration in cell chemistry must be reflected in the ability of the cells to divide in the presence of 2
H2
O and in the manner of its division. However, a many statements suggesting that 2
H2
O has a specific action on cell division are common since today. Probably it may be true that rapidly proliferating cells are highly sensitive to 2
H2
O, but that deuterium acts only to prevent cell division is unlikely.
The rabbit cells grown on medium containing the various concentrations of 2
H2
O shown, that 2
H2
O caused a reduction in cell division rate, and this effect increased as the concentration of 2
H2
O or duration of exposure, or both, were increased (Lavillaureix et all., 1962)
. With increasing concentration of 2
H2
O the frequency of early metaphases increased, accompanied by proportional decreases in the other phases.
It was suggested that 2
H2
O blocks mitosis in the prophase and the early metaphase of many cells grown in 2
H2
O. The blockage, however, was overcome if the initial concentration of 2
H2
O was not too high and the exposure time not too long. In experiments with eggs of the fresh water cichlid fish Aequidens portalegrensis
, they observed that in 30% 2
H2
O only one-fifth of the eggs hathed and in 50% (v/v) 2
H2
O none did so. Segmentation in fertilized frog eggs developed normally for 24 hours in 40% (v/v) 2
H2
O, after which the embryos died. It was also found by Tumanyan and Shnol
that 2
H2
O disturbed embryogenesis in Drosophila melanogaster eggs
(Lavillaureix et all., 1962
. Feeding female flies with 20% (v/v) 2
H2
O caused a significant increase in the proportion of nondeveloped eggs, whether males were deuterated or not.
As pointed out by many researches, carried elsewhere, the reason for the cessation of mitotic activity from exposure to 2
H2
O is not clear. Certain microorganisms have been adapted to grow on fully deuterated media. However, higher plants and animals resist adaptation to 2
H2
O. Even in microorganisms, however, cell division appears initially to be strongly inhibited upon transfer to highly deuterated media.
After the adaptation, however, cellular proliferation proceeds more or less normally in 2
H2
O, but this stage is not reached in higher organisms. No ready explanation in terms of the present understanding of mitosis suggests itself. In Arbacia
eggs antimitotic action of 2
H2
O is manifested almost immediately at all stages of the mitotic cycle and during cytokinesis (Gross P. R., et all., 1963, 1964
).
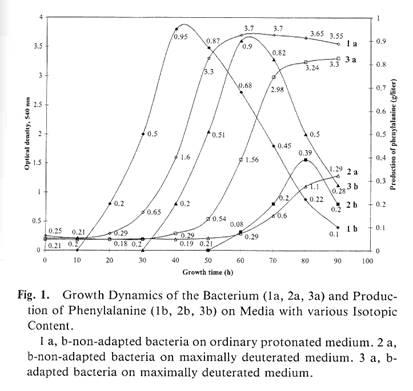
Table.
Isotope components of growth media and characteristics of bacterial growth of Brevibacterium methylicum
  |
Media components, % (v/v)
H2
O 2
H2
O MetOH [U -2
H]
MetOH
|
Lag-
phase (h) |
Yield of
biomass (%)
|
Generation time (h) |
Production of phenylalanine (%) |
| (a) |
98 |
0 |
2 |
0 |
20 |
100.0 |
2.2 |
100.0 |
| (b) |
73.5 |
24.5 |
0 |
2 |
34 |
85.9 |
2.6 |
97.1 |
| (c) |
49.0 |
49.0 |
0 |
2 |
44 |
60.5 |
3.2 |
98.8 |
| (d) |
24.5 |
73.5 |
0 |
2 |
49 |
47.2 |
3.8 |
87.6 |
| (e) |
0 |
98.0 |
0 |
2 |
60 |
30.1 |
4.9 |
37.0 |
A stabilizing action on the nuclear membrane and gel structures, i.e., aster, spindle, and peripheral plasmagel layer of the cytoplasm, can be detected. Prophase and metaphase cells in 80% (v/v) 2
H2
O remain frozen in the initial state for at least 30 minutes. Furrowing capacity probably is not abolished by 2
H2
O. The 2
H2
O-block is released on immersion in 2
H2
O although cells kept in deuterium-rich media for long periods show multipolar and irregular divisions after removal to 2
H2
O, and may subsequently cytolyze. The inhibition of mitosis in the fertilized egg is not the only interesting effect of deuterium. The unfertilized egg also responds. It was described by Gross
that deuterium parthenogenesis in Arbacia
in the following graphic terms: if an unfertilized egg is placed in 2
H2
O, there appear in the cytoplasm, after half an hour, a number of cytasters. The number then increases with time. If, after an hours immersion in 2
H2
O, eggs are transferred to normal sea water, a high proportion (80% of the population) raises a fertilization membrane, which gives evidence that activation has occurred.
Deuterium genetics is, for the most part, like genetics itself, conveniently divisible into dipteran mutation studies, the genetics of microorganisms, and miscellaneous studies of which those of Gross and Harding, and Flaumenhaft et al.
are examples. The customary procedure in most of the dipteran and bacterial investigations so far reported has been to administer 2
H2
O to the organism and then to test it for mutation or other chromosomal change. The results obtained by such an investigation have seldom been striking. For example, many researchers found an increase in sex-linked lethals in the sperm of flies that had been exposed to deuterium, either by way of injection into their pupae, or by the inclusion of 2
H2
O in their food. They introduced 2
H2
O into Drosophila melanogaster
larvae both by feeding and by injection. The males which matured from these larvae were tested for mutation by CIB method. But the test showed no increase in the mutation rate. It was assumed by these scientists that the deuterium which was used in dilute form entered the DNA molecule.
De Giovanni and Zamenhof
have carried out the most comprehensive investigations on the genetic effects of deuterium in bacteria. The results are of considerable interest. For example, they found a several mutants of E. coli
, including a so called rough mutant 1/D which is more resistant to 2
H2
O than its parent strain, were isolated from E. coli
grown in 2
H2
O media. The spontaneous frequency of occurerence of this mutant was 10-4
, and the mutation rate could be increased 300-fold by ultraviolet irradiation. This mutant was derived only from the strain E. coli
15 thymidine, and no similar mutant was observed in other strains of E. coli
or B. subtilis
. By application of a fluctuation test, De Giovanni
then was able to show convincingly that this mutation to increased deuterium resistance occurred spontaneously and not in response to the mutagenic effect of2
H2
O. Back mutations in some instances do seem to occur at higher rates in 2
H2
O. Reversion from streptomycin dependence to streptomycin sensitivity in E. coli
strain Sd/4, or from thymine dependence to thymine independence in strain 1 occurs with higher frequency in 2
H2
O, but 2
H2
O does not cause a discernible increase in mutation in the wild type.
De Giovanni
further found that deuteriated purines and pryrimidines had no effect upon the growth and back mutation rates of specific base-requiring strains. Thymine containing deuterium in two of the four nonexchangeable positions adequately supplied the requirement for thymine with no concominant genetic changes. It would appear therefore that the preponderance of the evidence from these studies with bacteria is in favor of the view that 2
H2
O is not a strong mutagenic agent.
It was reported by many researchers a series experiments designed to test the ability of deuterium to produce mutation and nondisjunction. Deuterium like tritium appear to increase nondisjunction, but either agent separately is less effective than the two acting together. Hughes
and Hildreth
exposed male flies which had been grown on a 20% (v/v) 2
H2
O diet to an irradiation of 1000 r. of X-rays. It was found that there was not significant difference in the frequency of observed mutations between 2
H2
O flies and normal flies subjected to the same radiation.
Tumanyan
and Shnol
also found no mutagenic effect of 2
H2
O on recessive and dominant lethal marks in D. melanogaster
, inbred line Domodedovo 18. Flaumenhaft
and Katz
grew fully deuteriated E. coli
in 99,6% (v/v) 2
H2
O with fully deuteriated substrates, and found that the mutation rate after ultraviolet irradiation was distinctly lower than that of nondeuteriated organisms. The simultaneous presence of both deuterium and protium in nearly equal proportions in the constituent molecule of an organism could conceivably create difficulties for the organism since the rate pattern would be seriously distorted. They further found that cells grown in 2
H2
O and then transferred to 2
H2
O showed an enhanced susceptibility to ultraviolet irradiation. This suggests that organisms containing both hydrogen or deuterium, but it leaves unanswered the question of why serial subculture in H2
O-2
H2
O media is required for adaptation of many organisms.
Many researchers studied the growth of phage T4
in E. coli
cells which were cultivated in media containing various concentrations of 2
H2
O from zero to 95% (v/v). No significant increase in forward mutation in this phage could be observed, but the rate for reverse mutation was increased, and reached a maximum in phage grown in 50% (v/v) 2
H2
O. Although it was reported that a further increase in H2
O concentration up to 90% (v/v) producers little augmentation of the reversion index, the actual data presented by Konrad
indicates a decided increase in reverse mutation rate in phage exposed to more than 50% (v/v) 2
H2
O.
There have been carried out a big deal of cytochemical study of fully deuteriated microorganisms grown autotrophically for very long periods in 2
H2
O (Flaumenhaft E., Conrad S. M., and Katz J. J., 1960a, 1960b
). The main conclusion that could be made from these studies is that the nucleus of deuterated cells was much larger than that of nondeuterated cells, and it contained greater amounts of DNA. Also present were much greater amounts of rather widely scattered cytoplasmic RNA within the cells. It was found also, that deuterated cells stained much more darkly for proteins, indicating higher concentrations of free basic groups. Both fluorescence and electron microscopy indicated that deuteration results in readily observable morphological changes. For example, the chloroplast structure of deuteriated plants organisms was more primitive in appearance, less well-differentiated, and distinctly less well-organized. The very interesting conclusion was made, then a low or/and high temperature grown organisms implied the morphological consequences of extensive isotopic replacement of hydrogen by deuterium so that in some respects resemble with the effects produced by reduction or/and increase in temperature of growth.
But, paradoxically as shown numerious studies on biological adaptation to 2
H2
O, a many cells of bacterial and algae origin could, nevertheless, well grown on absolute 2
H2
O and, therefore, to stabilize their biological apparatus and the structure of macromolecules for working in the presence of 2
H2
O. The mechanism of this stabilization nor at a level of the structure of [U-2
H]labeled macromolecules or at a level of their functional properties is not yet complitely understood. We still don’t know what possibilities a cell used for adaptation to 2
H2
O. We can only say, that probably, it a complex phenomenon resulting both from the changes in structural and the physiological level of a macrosystem. That is why there is every prospect that continued investigation of deuterium isotope effects in living organisms will yield results of both scientific and practical importance, for it is precisely. For example, the studies of the structure and the functioning of biolodical important [U -2
H]labeled macromolecules obtained via
biological adaptaition to high concentrations of 2
H2
O are most attract an attention of medical scientists as a simple way for creating a fully deuterated forms of DNA and special enzymes could well be working in a certain biotechnological processes required the presence of 2
H2
O. Secondly, if the structure of fully deuterated proteins may be stabilized in 2
H2
O in a view of duarability of deuterated bonds, it would be very interesting to study the thermo-stability of [U -2
H]labeled proteins for using them directly in processes going at high temperatures.
It would be very perspective if someone could create the thermo-stable proteins simply via
deuteration of the macromolecules by growing a cell-producent on 2
H2
O wit 99% 2
H. Third, particular interest have also the studies on the role of primodial deuterium in molecular evolution. The solution of these obscure questions concerning the biological adaptation to 2
H2
O should cast a new light on molecular evolution in a view of the preferable selection of macromolecules with difined deuterated structures. Thus, the main purpose of the present project is the studies of the structure and the function of fully deuterated macromolecules (particularly DNA and individual proteins and/or enzymes) obtained via biological adaptation to high concentrations of 2
H2
O.
To carry out the studies with fully deuterated macromolecules one must firstly to obtain the appropriate deuterated material with high level of enrichment for isolation of pure DNA and individual proteins to whom the various methods of stable isotope detection further can be applyed. For example, the three-dimentional NMR combined together with the method of X-ray diffraction, infrared (IR)-, laser spectrometry and circular dichroism (CD) is a well proved method for the studies of the structure and the functioning of [U -2
H]labeled macromolecules, and for investigations of various aspects of their biophysical behavior. Taking into account the ecological aspect of using [U -2
H]labeled compounds, it should be noted in conclusion, that the preferable properties of applying deuterium for biochemical studies are caused mainly by the absence of radioactivity of deuterium that is the most important fact for carrying out the biological incorporation of deuterium into organism.
2. SCIENTIFIC ACTUALITY OF THE RESEARCH
A special attention is to be given to the investigation of biological adaptation to 2
H2
O allowing cells to synthesize a deuterated forms of macromolecules (particulary interest have DNA and short-chain individual proteins both with well known amino acid sequence and conformation) with a certain structure allowing their functioning in 2
H2
O environment.
Firstly
, in this connection it would be very interesting to know, how the structure of fully deuterated macromolecules could be changed neganively or positively in a course of biological adaptation to 2
H2
O requiring the presence of high concentrations of 2
H2
O in growth media.
Secondly
, if a cell will be growing on media containing the stepwise increasing concentrations of 2
H2
O, for example starting up from zero up to 100% (v/v) 2
H2
O, will the changes in the structure of [U -2
H]labeled macromolecules to be corresponding to the 2
H2
O content in media and what is a limit concentration of 2
H2
O when the macromolecular structure keeps a stable constancy and how this fact corresponds with a limit of biological resistance to 2
H2
O? For answers to these questions a number of modern consideration at the levels of the structure (primary, secondary, tertiary) and conformation of [U -2
H]labeled DNA and individual proteins with using the methods of a special sequencing and modifications of deuterated macromolecules combined together with gel electrophoresis method as well as such powerful methods as NMR-spectroscopy to which will be taken a most part of proposed research, X-ray diffraction, IR-, laser- and CD-spectroscopy will be further involved.
An investigation will necessary mainly into the structure of [U -2
H]labeled macromolecules in order to find at what level of macromolecular hierarchy a substitution of hydrogen atoms with deuterium ensued the consequence on the differences in the structure and the conformation of macromolecules and, therefore, the functional properties of the macromolecules in 2
H2
O. In the frames of proposed research the developing of methods of biological adaptation to obtain [U -2
H]labeled biological material with high levels of enrichment are also of a big interest. For this purpose the special biotechnological approaches based on using the strains with improved properties when growing on 2
H2
O for obtaining fully deuterated DNA and individual proteins should be applied for allowing to prepare [U -2
H]labeled macromolecules in gram scale quantities.
3. DISCUSSION
3.1.
The methods for analyzing the structure and the conformation of
[U -2
H]labeled
macromolecules.
The biological labelling with deuterium is an useful tool for investigating the structure and the conformational properties of macromolecules. The fundamental objectives have meant that living models have retained their importance for functional studies of such biological important macromolecules and can be used to obtain structural and dynamic information about the [U -2
H]labeled macromolecules.
The method of X-ray diffraction should be noted as a indespencible tool for determing the details of the three-dimentional structure of globular proteins and other macromolecules (Mathews C. K., van Holde K. E., 1996
). Yet this technique has the fundamental limitation that it can be employed only when the molecules are crystallized, and crystallization is not always easy or even possible. Furthermore, this method cannot easily be used to study the conformational changes in response to changes in the molecules environment.
Other methods, for example IR-spectroscopy, can provide direct information concerning the macromolecular structure. For example, the exact positions of infrared bands corresponding to vibrations in the polypeptide backbone are sensitive to the conformational state (a helix, b sheet et.) of the chain (Campbell I. D., and Dwek R. A., 1984
). Thus, the studies in this region of the spectrum are often used to investigate the conformations of protein molecules.
Although, IR-, and absorption spectroscopy can be helpful in following molecular changes, such measurements are difficult to interpret directly in terms of changes of secondary structure. For this purpose, techniques of circular dichroism involving polarized light have become important (Johnson W. C., 1990
). For example, if a protein is denatured so that its native structure, containing a helix and b sheet regions, is transformed into an unfolded, random-coil structure, this transformation will be reflected in a dramatic change in its CD spectrum. Circular dichroism can be used in another way, to estimate the content of a helix and b sheet in native proteins. The contributions of these different secondary structures to their circular dichroism at different wavelenghths are known, so we may attempt to match an observed spectrum of protein by a combination of such contributions.
Although circular dichroism is an extremely useful technique, it is not a very discriminating one. That is, it cannot, at present, tell us what is happening at a particular point in a protein molecule. A method that has the great potential to do so is nuclear magnetic resonance. This advance now make it possible to use NMR to study a big varieties of DNA and proteins with more complex biological functions functioning in natural liquid environment. Often these proteins have more than one domain and more than one site of interaction. Allosteric systems, receptors and small molecule ligand-modulated DNA-binding proteins and DNA are some examples of the molecular systems which can now be analysed in molecular detail. For example, due to the development of two-dimentional Fourier transformation techniques, NMR spectroscopy has become a powerful tool for determining the protein structure and conformation (Fesic S. W. and Zuiderweg E. R., 1990
).
3.2. The preparation of [U- 2
H]labeled macromolecules.
Through technical advances of biotechnology, many macromolecules, for example a certain individual proteins are successfuly cloned and can be obtained in large quantities by expression in microbial and/or mammalian systems, so that an ever-increasing number of individual [U- 2
H]labeled macromolecules from various biological objects are becoming commercially available. It should be noted, however, that the application of various methods for the preparation of [U -2
H]labeled macromolecules (chemical or biosynthetical) often results in obtaining the forms of molecules with different number of protons substituted by deuterium, the phenomenon that is known as heterogenious labelling, so that the special methods for the preparation of [U -2
H]labeled macromolecules should be applyed to minimaze this process. For example, the proteins containing only deuterium atoms in polypeptide chain of macromolecule can be produced biotechnologically with using the special genetically constructed strains of bacteria carrying the mutations of geens excluding the metabolic exchange between the parterns of unlabeled intermediators during the biosynthesis of [U -2
H]labeled macromolecules.
I may briefly indicate three possibilities for deuterium enrichment:
(1) to grow the organism on a minium salt medium with content of 2
H2
O 99% 2
H;
(2) To grow the organism on a medium supplemented with 99% 2
H2
O and [U -2
H]labeled amino acid mixture.
(3) the isotopic exchange of susceptible protons in amino acid residues already incorporated into protein.
Method 1 is very useful for the preparation of [U- 2
H]labeled macromolecules if only applyed strains of bacterial or different origin could well be grown on minimal media in the presence of high concentrations of 2
H2
O. Very often in this case the biological adaptation to 2
HO is required. Method 2, while generally applicable, is limited by the difficulty and expense of preparing fully deuterated amino acid mixtures from algae grown on 2
H2
O. However, recently we proposed to use a fully deuterated biomass of methlotrophic bacterium B. methylicum
with protein content about 55% (from dry weight) obtained via
multistep adaptaition to 98% (v/v) 2
H2
O and 2% (v/v) [U-2
H]MetOH as growth substrates for growing the other bacterial strains to prepare a gram quantities of [U -2
H]labeled amino acids, proteins and nucleosites with high levels of enrichment (90.0-97.5% 2
H) (Mosin O. V., Karnaukhova E. N., Pshenichnikova A. B.; 1994; Skladnev D. A., Mosin O. V., et all; 1996; Shvets V. I., Yurkevich A. M., Mosin O. V.; 1995).
Method 2 is also necessary when the organism will not grow on a minimal medium as it was in the case with the applying the bacteria requiring the complex composition media for their growth. This approach will also be necessary for the labeling of proteins expressed in systems other than E. coli
(e.g
. yeast, insect, and mammalian expression systems) which may be important for the proper folding of proteins from higher organisms. Since the protons of interest in proteins are most often carbon bound and thus do not exchange under mild conditions, method 3 is severely limited by stability of proteins under the harsh conditions necessary for (1
H-2
H) exchange.
4. ADAPTATION TO 2
H2
O AND BIOPHYSICAL PROPERTIES OF [U -2
H]LABELED MACROMOLECULES
FIGURE
The imaginary principle of realization of biological adaptation
I
II
  1 works 2 not work not work 2 works 1 works 2 not work not work 2 works
     
 
ordinaryenvironment(A) 2
H2
O (B)
4.1. The main hypothese
.
Weproposed that a cell theoretically could in principle synthezise a big number of forms of [2
H]labeled macromolecules with somewhat different structures and conformations, so that a cell could easily select a preferable one from al these species in a course of adaptation to 2
H2
O, that is the best suitable namely for that conditions. A simple imaginary principle I am going to discuss here perhaps somewhat may explain this probable mechanism. Let us suppose, for example that there are at least two imadinary structural systems - ordinary (normal) system call it a system 1 and unordinary (adaptive) system 2 (see a Figure above). Supporse, that the environment is a homoginious substanse and compose from ordinary substance A (H2
O) (situation 1). The necessarely condition for the normal working of this model in natural H2
O environment is that system 1 works and system 2 stay in background (situation 2). Supporse, that the environment have changed for substance B (2
H2
O). Then the system 2 will work, while the system 1 will stay in background (situation 2). When environment will be the natural again, the system 1 will begin the work again, while the system 2 will stay in background. Admitt, that the two systems both presented at the time being and could be regulated in such way that they may switch bitween each other during the working so that the model system does not undergoing the considerable alterations.
4.2. Phenomenon of biological adaptation to 2
H2
O
.
Our research has confirmed,that ability to adaptation to 2
Н2
О is differed for various species of bacteria and can to be varried even in frames of one taxonomic family (Mosin O. V. et al., 1996a, 1996b
).From this, it is possible to conclude, that the adaptation to 2
Н2
О is determined both by taxonomic specifity of the organism, and peculiarities of the metabolism, as well as by functioning of various ways of accimilation of hydrogen (deuterium) substrates, as well as evolutionary level, which an object itself occupies. The less a level of evolutionary development of an organism, the better it therefore adapts itself to 2
H2
O. For example, there are halophilic bacteria that are being the most primitive in the evolutionary plan, and therefore, they practically not requiring to carry out a special adaptation methods to grow on 2
Н2
О. On the contrary, bacills (eubacteria) and methylotrophs (gram-negative bacteria) worse adapted to 2
Н2
О.
At the same time for all tested cells the growth on 2
H2
O was accompanied by considerable decrease of a level of biosynthesis of appropriated cellular compounds. The data obtained confirm that the adaptation to 2
Н2
О is a rather phenotypical phenomenon, as the adapted cells could be returned to a normal growth and biosynthesis in protonated media after lag-
phase (Mosin O. V. et al., 1993)
.
However, when the adaptive process goes continuously during the many generation, the population of cells can use a special genetic mechanisms for the adaptation to 2
H2
O. For example, mutations of geens can be resulted in amino acid replacements in molecules of proteins, which in turn could cause a formation of a new isoenzymes, and in the special cases - even the anomal working enzymes of a newer structure type. The replacements of these compounds can ensure a development of new ways of regulation of enzymic activity, ensuring more adequate reaction to signals, causing a possible changes in speeds and specifity of metabolic processes.
Despite it, the basic reactions of metabolism of adapted cells probably do not undergo essential changes in 2
Н2
О. At the same time the effect of convertibility of growth on Н2
О/2
Н2
О - does not theoretically exclude an opportunity that this attribute is stably kept when cells grown on 2
Н2
О, but masks when transfer the cells on deuterated medium.
However, here it is necessary to emphasize, that for realization of biological adaptation to 2
H2
O the composition of growth medium plays an important role. In this case it is not excluded, that during the adaptation on the minimal medium, containing 2
Н2
О there are formed the forms of bacteria, auxotrophic on a certain growth factors (for example amino acids et) and thereof bacterial growth is inhibited while grown on these media. At the same time the adaptation to 2
Н2
О occurs best on complex media, the composition of which coul compensate the requirement in those growth factors.
It is possible also to assume, that the macromolecules realize the special mechanisms, which promote a stabilization of their structure in 2
H2
O and the functional reorganization for best working in 2
Н2
О. Thus, the distinctions in nuclear mass of hydrogen atom and deuterium can indirectly to be a reason of distinctions in synthesis of deuterated forms of DNA and proteins, which can be resulting in the structural distinctions and, hence, to functional changes of [2
H]labeled macromolecules. Hawever, it is not excluded, that during incubation on 2
Н2
О the enzymes do not stop the function, but changes stipulating by isotopic replacement due to the primary and secondary isotopic effects as well as by the action of 2
Н2
О as solvent (density, viscosity) in comparison with Н2
О are resulted in changes of speeds and specifics of metabolic reactions.
In the case with biological adaptation to 2
H2
O we should inspect the following types of adaptive mechanisms:
1. adaptation at a level of macromolecular components of cells:
It is possible to allocate mainly two kinds of such adaptation:
(a). Differences of intracellular concentration of macromolecules;
(b). The forming in 2
H2
O the deuterated macromolecules with other conformations, which could be replaced the ordinary protonated macromolecules synthesized by cells in normal conditions.
We suppose, that in principle, any protein macromolecule could adopt an almost unlimited number of conformations. Most pilypeptide chains, however, fold into only one particular conformation determined by their amino acid sequence. That is because the side chains of the amino acids associate with one another and with water (2
H2
O) to form various weak noncovalent bonds. Provided that the appropriate side chains are present at crucial positions in the chain, large forces are developed that make one particular conformation especially stable.
These two strategies of adaptation could possible to be distinqueshed accordinly as "quantitative
" and "qualitative"
strategies;
2. adaptation at a level of microenvironment in wich macromolecules are submerged:
the essence of this mechanism is, that the adaptive change of structural and conformational properties of [2
H]labeled macromolecules is conditioned both by directional action of 2
H2
O environment on a growth of cells and by its physico-chemical structure (osmotic pressure, viscosity, density, рН, concentration of 2
H2
O).
2
H2
O appeared to stabilize the plasmagel structure of biological microenvironment. The external pressure required to make the cells assume a spherical shape increased 3.6 kg/cm2
for each per cent increase in the presence of 2
H2
O. It thus seems well established that deuteration can affect the mechanical properties of cytoplasm, and that this factor must be taken into account in assessing the consequences of isotopic substitution of macromolecules. In model experiments with gelatin structure, it was demonstrated that in 2
H2
O there is a greater protein-protein interaction than in H2
O (Scheraga J. A; 1960)
.
A progressive increase in the melting temperature of the gel in 2
H2
O is observed accompanied by an increase in the reduced viscosity. That 2
H2
O can have marked effects on the physical properties of proteins has been known for some time. Consequently it is natural to attribute changes in the mechanical properties of cell structures induced by 2
H2
O to protein response. Nevertheless, the effects of deuterium on proteins, while real, must be only a partial explanation of the situation. The interaction of proteins with solvent water is extraordinarily complex, and the exact nature of the protein is crucial in determining the magnitude of changes resulting from the replacement of H2
O by 2
H2
O.
This mechanism has extremely large importance and supplements the macromolecular adaptation; 3. adaptation at a functional level
, when the change of an overall performance of macromolecular systems, is not connected with a change of a number of macromolecules being available or with the macromolecules of their types. Adaptation in this case could provide the changes by using the already existing macromolecular systems - according to requirements by this or that metabolic activity.
TABLE
Some physical constants of ordinary and heavy water
Physical constant
|
H2
O
|
2
Н
2
О
|
| Density, d
20
(g/c.c) |
0,9982 |
1,1056 |
| Molecular volume, V
20
(ml/mole) |
18,05 |
18,12 |
| Viscosity m20
(centipose) |
1,005 |
1,25 |
| Melting point (0
C) |
0,1 |
3,82 |
| Boiling point (0
C) |
100,0 |
101,72 |
| Temperature of maximum density (0
C) |
4,0 |
11,6 |
| Ion product (25 0
C) |
10-14
|
0,3x10-14
|
| Heat of formation (cal/mole) |
-68,318 |
-70,414 |
| Free energy of formation (cal/mole) |
-56,693 |
-58,201 |
| Entropy (e.u/mole) |
45,14 |
47,41 |
Secondary effects may still be of importance in biological systems sensitive to kinetic distortions. Deuterium also affects equilibrium constants, particularly the ionization constants of weak acids and bases in composition of macromolecules dissolved in heavy water (see a Table below). Acid strength of macromolecules in 2
H2
O is decreased by factors of 2 to 5, and consequently, the rates of acid-base catalyzed reactions may be greatly different in 2
H2
O as compared to H2
O. Such reactions frequently may be a faster in 2
H2
O than H2
O solution (Covington A. K., Robinson R. A., and Bates R. G., 1966; Glasoe P. K., and Long F. A., 1960
).
4.2. The chemical isotopic effect of 2
H2
O
.
The effect of isotopic replacement that has particularly attracted the attention of chemists is the kinetic isotope effect (Thomson J. F., 1963).
The substitution of deuterium for hydrogen in a chemical bond of macromolecules can markedly affect the rate of scission of this bond, and so exert pronounced effects on the relative rates of chemical reactions going in 2
H2
O with participation of macromolecules. This change in rate of scission of a bond resulting from the substitution of deuterium for hydrogen is a primary isotopic effect. The direction and magnitude of the isotope effect will depend on the kind of transition state involved in the activated reaction complex, but in general, deuterium depresses reaction rates. The usual terminology of the chemist to describe the primary kinetic effect is in terms of the ratio of the specific rate constants kh/kd
. The maximum positive primary kinetic isotopic effect which can be expected at ordinary temperatures in a chemical reaction leading to rupture of bonds involving hydrogen can be readily calculated, and the maximum ratio kh/kd
in macromolecules is in the range of 7 to 10 for C-H versus C-2
H, N-H versus N-2
H, and O-H versus O-2
H bonds. However, maximum ratios are seldom observed for a variety of reasons, but values of kh/kd
in the range of 2 to 5 are common (Wiberg K. B., 1955
). Deuterium located at positions in a macromolecule other than at the reaction locus can also affect the rate of a reaction. Such an effect is a secondary isotope effect and is usually much smaller than a primary isotope effect.
In general, when the macromolecules transfer to deuterated medium not only water due to the reaction of an exchange (Н2
О -2
Н2
О) dilutes with deuterium, but also occurs a very fast isotopic (1
Н-2
Н)-exchange in hydroxylic (-OH), carboxilic (-COOH), sulfurhydrilic (-SH) and nitrogen (-NH; -NH2
) groups of all organic compounds including the nucleic acids and proteins. It is known, that in these conditions only С-2
Н bond is not exposed to isotopic exchange and thereof only the species of macromolecules with С-2
H type of bonds can be synthesized de novo. This is very probably, that the most effects, observed at adaptation to 2
Н2
О are connected with the formation in 2
Н2
О [U -2
H]labeled molecules with conformations having the other structural and dynamic properties, than conformations, formed with participation of hydrogen, and consequently having other activity and biophysical properties.
So, according to the theory of absolute speeds the break of С-1
H-bonds can occur faster, than С-2
H-bonds (C-2
H-bonds are more durable than C-1
, mobility of an ion 2
H+ is less, than mobility of 1
Н+, the constant of ionization 2
Н2
О is a little bit less than ionization constant of 2
Н2
О. Thus, in principle, the structures of [U -2
H]labeled macromolecules may to be more friable that those are forming in ordinary H2
O. But, nevertheless, the stability of [U -2
H]labeled macromolecules probably depending on what particular bond is labeled with deuterium (covalent bonds -C2
H that causing the instability or hydrogen bonds causing the stabilization of conformation of macromolecules via
forming the three-dimentional netwok of hydrogen(deuterum) bonds in macromolecule) and what precise position of the macromolecule was labeled with deuterium. For example, the very valuable and sensitive for deuterium substitution position in macromolecule is the reactive center (primary isotopic effects). The non-essential positions in macromolecule are those ones that situated far away from the reactive center of macromolecule (secondary isotopic effects). It is also possible to make a conclusion, that the sensitivity of various macromolecules to substitution on 2
Н bears the individual character and depending on the structure of macromolecule itself, and thus, can be varried. From the point of view of physical chemistry, the most sensitive to replacement of 1
Н+ on 2
H+ can appear the apparatus of macromolecular biosyntesis and respiration system, those ones, which use high mobility of protons (deuterons) and high speed of break of hydrogen (deuterium) bonds. From that it is posible to assume, that the macromolecules should realize a special mechanisms (both at a level of primary structure and a folding of macromolecules) which could promote the stabilizition of the macromolecular structure in 2
H2
O and somewhat the functional reorganization of their work in 2
H2
O.
A principal feature of the structure of such biologically important compounds as proteins and nucleic acids is the maintenance of their structure by virtue of the participation of many hydrogen bonds in macromolecule. One may expect that the hydrogen bonds formed by of many deuterium will be different in their energy from those formed by proton. The differences in the nuclear mass of hydrogen and deuterium may possibly cause disturbances in the DNA-synthesis, leading to permanent changes in its structure and consequently in the cells genotype. The multiplication which would occur in macromolecules of even a small difference between a proton and a deuteron bond would certainly have the effect upon its structure.
The sensitivity of enzyme function to structure and the presumed sensitivity of nucleic acids function (genetic and mitotic) to its structure would lead one to expect a noticeable effect on the metabolic pattern and reproductive behavior of the organism. And next, the changes in dissociation constants of DNA and protein ionizable groups when transfer the macromolecule from water to 2
H2
O may perturb the charge state of the DNA and protein. Substitution of 1
H for deuterium also affects the stability and geometry of hydrogen bonds in apparently rather complex way and may, through the changes in the hydrogen bond zero-point vibrational energies, alter the conformational dynamics of hydrogen (deuterium)-bonded structures within the DNA and protein in 2
H2
O.
5. CONCLUSION
The successful adaptation of organisms to high concentration of 2
H2
O will open a new avenues of investigation with using [U- 2
H]labeled macromolecules could be isolated from these organisms. For example, fully deuterated essential macromolecules as proteins and nucleic acids will give promise of important biological, medical and diagnostical uses. Modern physical methods of study the structure of [U- 2
H]labeled macromolecules, particularly three-dimentional NMR in a combination with crystallography methods, X-ray diffraction, IR-, and CD- spectroscopy should cast new light on many obscure problems concerning with the biological introduction of deuterium into molecules of DNA and proteins as well as the structure and the function of macromolecules in the presence of 2
H2
O. The variety of these and other aspects of biophysical properties of fully deuterated macromolecules in the presence of 2
H2
O remain an interesting task for the future.
First
,
I hope that the structural and the functional studies of [U- 2
H]labeled macromolecules can provide us to the useful information about a many aspects of the synthesis of fully deuterated macromolecules and their biophysical behaviour in 2
H2
O.
Second
,
the extensive body of available structural data about a cell protection system (at the level of the structure and the functioning of [U- 2
H]labeled DNA and enzymes) will also form the basis for a particularly useful model for the study of biological adaptation to 2
H2
O in aspect of molecular evolution of macromolecules with difined isotopic structures.
Finally
, we also believe, the research can make a favour the medicine and biotechnology, especially for creating a fully deuterated analogues of enzymes and DNA having something different properties then the protonated species and working in the presence of 2
H2
O.
6. LITERATURE
Campbell I. D., and Dwek.
Biological Spectroscopy. Benjamin/Cummings, Menlo Park, Calif. 1990.
Covington A. K., Robinson R. A., and Bates R. G.
// J. Phys. Chem. 1966. V. 70. P. 3820.
Е
gorova T. A., Mosin O. V., Shvets V. I., et al.
// Biotechnologija. 1993. ¹.8. P. 21-25.
Fesic S. W. and Zuiderweg E. R.
// Quarterly Reviews of Biophysics. - 1990. - V.23. - N.2. - P. 97-131.
Johnson W. C.
Protein secondary structure and circular dichroism: A practical guide. Proteins Struct. Funct. Genet. 1990. 7:205-214.
Glasoe P. K., and Long F. A.
// J. Phys. Chem. 1960. V. 64. P. 188.
Hogan C. J.
// Scientific American. December 1996. P. 36-41.
Karnaukhova E. N., Mosin O. V., and Reshetova O. S.
// Amino Acids. 1993. V.5. ¹.1.P.125.
Katz J., Crespy H. L.
// Pure Appl. Chem. 1972. V. 32. P. 221-250.
Lewis G. N.
// Science. 1934. V. 79. P. 151.
Mathews C. K., van Holde K. E. Biochemistry
Benjamin/Cummings, Menlo Park, Calif. 1996. P. 204-210.
Mosin O. V., Karnaukhova E. N., Skladnev D. A., et al.
// Biotechnologija. 1993. ¹.9. P. 16-20.
Mosin O. V., Karnaukhova E. N., Pshenichnikova A. B., Reshetova O. S.
Electron impact spectrometry in bioanalysis of stable isotope labeled bacteriorhodopsin. in: Sixth International Conference on Retinal Proteins. 19-24 June 1994. Leiden. The Netherlands. P.115.
Mosin O. V., Karnaukhova E. N., and Skladnev D. A.
Preparation of 2
H-and 13
C-amino acids via bioconvertion of C1
-substrates. in: 8th
International Symposium on Microbial Growth on C1
Compounds. 27 August-1 September 1995. San Diego. U.S.A. P. 80.
Mosin O. V., Skladnev D. A., Egorova T. A., Yurkevich A. M., Shvets V. I.
// Biotechnologija. ¹3. 1996a. P. 3-12.
Mosin O. V., Egorova T. A., Chebotaev . B., Skladnev D. A., Yurkevich A. M., Shvets V. I.
// Biotechnologija. 1996b. ¹ 4. P. 27-34.
Mosin O. V., Kazarinova L. A., Preobrazenskaya K. A., Skladnev D. A., Yurkevich A. M., Shvets V. I.
// Biotechnologija. 1996c. ¹ 4. P. 19-26.
Mosin O. B., Skladnev D. A., Egorova T. A., Shvets V. I
// Bioorganicheskaja khimia. 1996d. V. 22. N 10-11. P. 861-874.
Skladnev D. A., Mosin O. V., Egorova T. A., Eremin S. V., Shvets V. I.
Methylotrophic bacteria as sourses of 2
H-and 13
C-amino acids. // Biotechnologija. ¹5. 1996. P. 14-22.
Shvets V. I., Yurkevich A. M., Mosin O. V., Skladnev D. A
// Karadeniz Journal of Medical Sciences. 1995. V.8. ¹ 4. P.231-232.
Thomson J. F.
Biological Effects of Deuterium. 1963. Pergamon, New York.
Tomita K., Rich A., de Loze C., and Blout E. R.
// J. Mol. Biol. 1962. V. 4. P. 83.
Wiberg K. B.
// Chem. Rev. 1955. V. 55. P. 713.
|